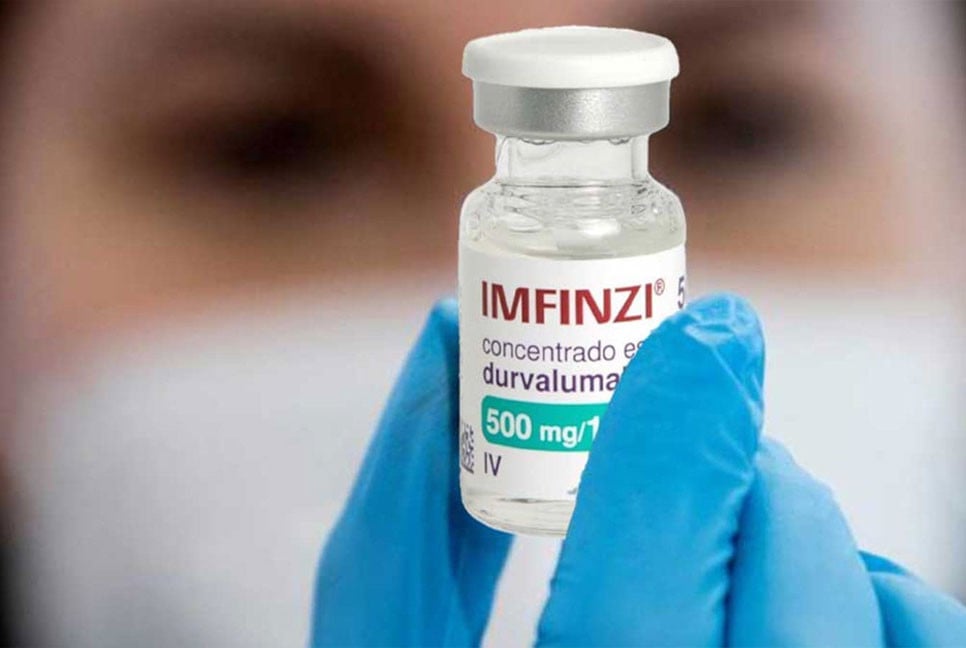
The World Health Organization (WHO) has raised an alert on Monday (Geneva local time) concerning falsified IMFINZI (durvalumab) injection 500mg/10ml, discovered in Armenia, Lebanon, and Türkiye. These counterfeit products, identified in the unregulated supply chain and reported to WHO in November 2024, pose significant risks to patient safety. Laboratory analysis by AstraZeneca, the genuine manufacturer, confirmed that the falsified products contain no active pharmaceutical ingredient, rendering them ineffective and potentially life-threatening.
IMFINZI is a monoclonal antibody used as a vital treatment for Non-Small Cell Lung Cancer (NSCLC) in adults. The falsified versions deliberately misrepresent their identity, composition, and source. Key indicators of these counterfeit products include incorrect lot numbers, mismatched manufacturing or expiry dates, and packaging inconsistencies such as misplaced data matrix codes, color discrepancies, and improperly crimped vial closures.
WHO stated, “These falsified products should be considered unsafe, and their use may be life-threatening in some circumstances. The use of these falsified IMFINZI products may lead to ineffective or delayed treatment. It is important to detect and remove any falsified IMFINZI (durvalumab) injections from circulation so as to prevent harm to patients.”
The use of these falsified IMFINZI injections may lead to ineffective treatment, delays in medical care, and life-threatening situations for cancer patients. WHO advises all healthcare professionals to immediately report adverse effects or suspected falsification to national regulatory authorities or pharmacovigilance centers. Patients in possession of these products are urged not to use them and to seek medical advice if exposed.
WHO recommends heightened surveillance in supply chains, particularly in unregulated markets, and calls on national authorities to notify WHO promptly if the counterfeit products are detected. Proactive measures are essential to protect patients and prevent further harm caused by these unsafe medications.
Bd-pratidin English/ Jisan